SOP for Validation/Qualification Policy in Pharmaceutical Industry
Objective: To lay down a procedure for validation/qualification policy for equipment, system and instruments. Scope: This procedure is applicable for production/engineering equipment, utilities and laboratory instruments. Responsibility:…



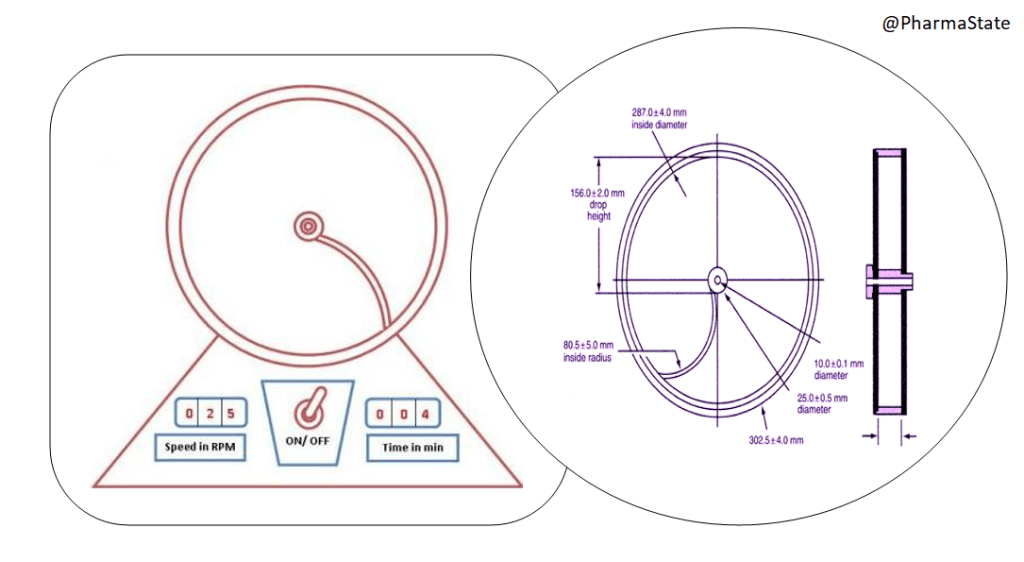
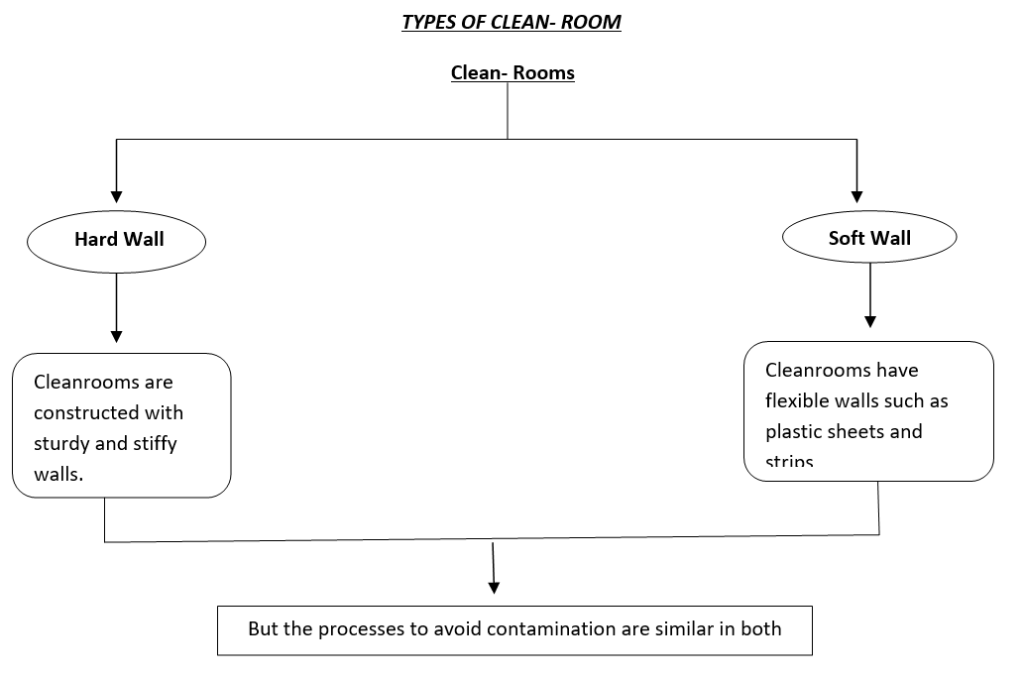
You must be logged in to post a comment.