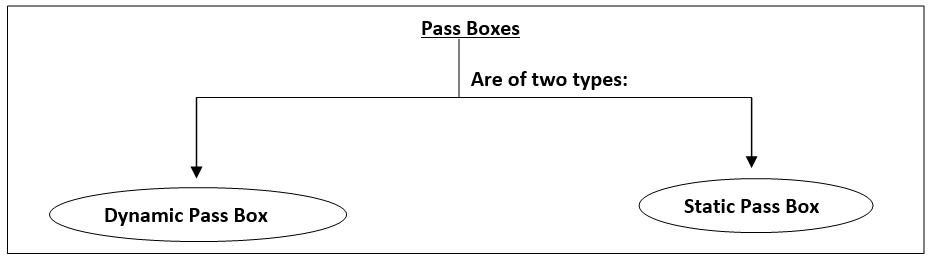
Important SOPs for Quality Control Department (Chemical section) in Pharmaceutical Industry
Sampling of raw material, packaging material, bulk, intermediate and finished product Raw material, packaging material, bulk, intermediate and finished product analysis Cleaning and storage of…




You must be logged in to post a comment.